Anthrax vaccine stability data delivers contract extension for Glide Technologies
In 2012, Pfenex asked UK-based Glide to formulate a recombinant protective antigen (rPA) using its solid dose injector (SDI) tech in a project funded by the US National Institute of Allergy and Infectious Diseases under the Government’s biodefence programme.
This work is now completed accord to Glide, which told us “the objective of the first phase of the product was to demonstrate stability, which has been successfully completed. We will move towards validation of performance of the vaccine in the next phase.”
The only vaccine approved in the US is Emergent Biosolutions’ product BioThrax which is made from immunogenic proteins harvested from the surface of the bacteria that causes anthrax, Bacillus anthracis.
BioThrax – also known as ASA – has been to protect veterinarians and others working with infected animals since the early 70s. In 1991, BioThrax’ use increased dramatically after the military administered it to soldiers serving in Iraq in a bid to protect against biological weapons.
Six shots required
Military use caused considerable controversy with some groups linking to a debilitating condition known as Gulf War Syndrome suffered by some Iraq veterans. Such links have been dismissed by the US IOM and the Department of Veterans Affairs.
But the negative publicity combined with old concerns about manufacturer Michigan Biologic Products Institute (MBPI) have tarnished BioThrax’ image.
Subsequently, while the vaccine is still used by the military, the US Government has encouraged efforts to develop alternatives. In 2004, the HSS ordered $877m worth of recombinant antigen vaccine from Vaxgen under Project BioShield.
This contract came to nothing in 2006 after the US Food and Drug Administration (FDA) refused to approve planned trials of the vaccine over concerns it was unstable. Two years later VaxGen sold the vaccine to Emergent Biosolutions.
Pfenex is one of a number of firms developing a recombinant anthrax vaccine including Emergent, Vaxin and Pharmathene.
Stability
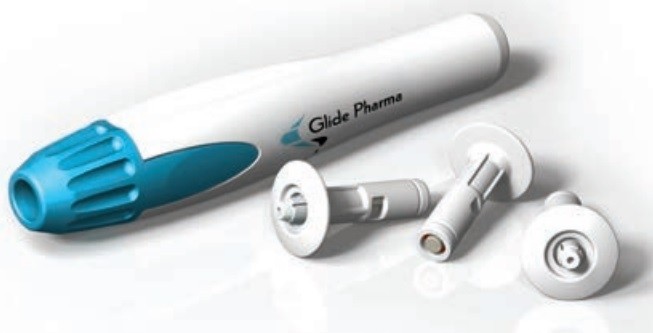
Stability enhancement is a major strength of the SDI technology according to Glide, which told us the proteins delivered with the solid dose injector system are formulated into solid dosages that are stable at room temperature.
“Glide’s technology comprises two key elements: Formulation of proteins into a solid dose; and delivery of the solid dose using an injector system. The formulated drug product is a small rod around 4mm long x 0.8mm diameter which is pushed through the skin.”