SolasCure ploughs ahead with maggot-derived wound gel after positive phase 2 results
The news comes after the company demonstrated initial proof-of-concept for its treatment at the end of 2023.
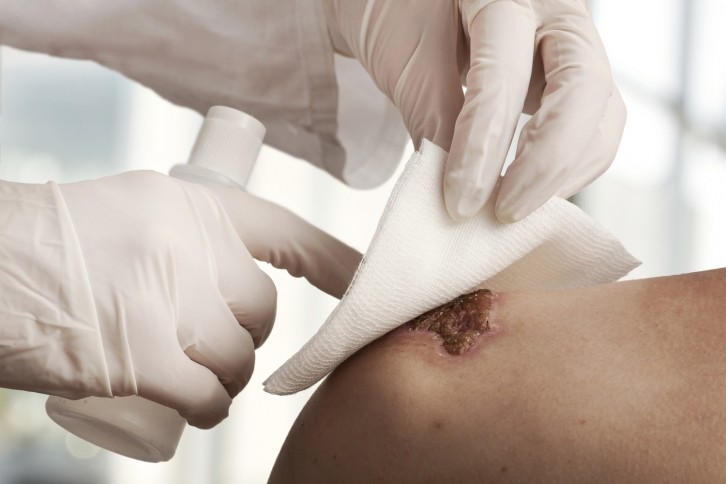
SolasCure's product is a hydrogel containing tarumase (provisional INN), a recombinant enzyme derived from maggots, which aims to accelerate healing and help wound bed preparation.
The phase 2 data has now been peer-reviewed and published, providing ‘strong validation’ as the company progresses into further clinical studies, and marks a ‘significant milestone’ for the biotech.
“The opportunity for aurase wound gel to truly transform chronic wound care is very exciting, as no other treatment to date aims to target all elements of wound care management in a single product,” said Andy Weymann, chairman of the board at SolasCure.
“The peer-review publication of our phase 2a data not only provides important validation to enable further phase 2 studies, but also highlights the clinical potential of aurase wound gel to treat millions of patients globally safely and effectively, addressing an urgent and unmet medical need. With this excellent data we are now fundraising to support the next phase of SolasCure’s clinical and product development.”
The study was performed in venous leg ulcer (VLU) patients across centers in the US, UK, and Hungary.
Tarumase was found to successfully debride wounds and demonstrated a strong safety profile, with no indications of systemic absorption, antibody generation, or systemic effects on coagulation.
Application of the gel was also shown to be pain-free, did not add to the patients’ existing pain burden, and had no evidence of local tolerability issues.
Chronic wounds are a significant healthcare challenge, with around 100 million people suffering globally.
In fact, recent clinical data from SolasCure claims that complete wound closure is only achieved in 25-50% of patients after 20 weeks of the current standard of care treatment.
The company hopes to address this challenge by being the first treatment to target all elements of wound bed preparation: debridement, moisture provision, infection control and overall promotion of healing.
“Debridement is a key first step of successful wound bed preparation, itself a prerequisite for wound healing,” added Rob Kirsner, head of medical advisory board at SolasCure and professor of dermatology at the University of Miami.
“Achieving timely complete and pain-free debridement which is agnostic of the patient setting is an urgent unmet medical need. Our gel has shown in this publication positive safety and proof-of-concept results, which bring this product a significant step closer to providing relief to those suffering from chronic wounds worldwide.”
Moving forward, the company is planning to conduct further phase 2 studies over a longer period, exploring the efficacy of tarumase at higher concentrations.