CureVac continues regulatory push for COVID-19 shot despite low efficacy
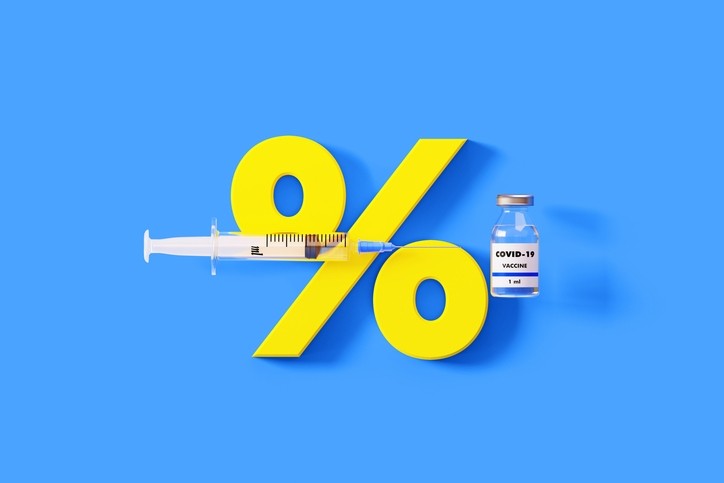
This is despite its latest data showing only 48% efficacy for the shot.
Yesterday [June 30], CureVac reported results of the final analysis of its 40,000 subject international pivotal Phase 2b/3 HERALD study of CVnCoV.
That trial involved around 40,000 participants in 10 countries in Latin America and Europe.
‘Increasingly diverse environment of COVID-19 variants’
CureVac said that, in the unprecedented context of 15 strains circulating within the study population at the time of final analysis, CVnCoV demonstrated an overall vaccine efficacy of 48% (vaccine 83 vs. 145 placebo) against COVID-19 disease of any severity, including single non-respiratory mild symptoms.
It noted significant protection was demonstrated among participants in the age group of 18 to 60, with an efficacy of 53% (vaccine 71 vs. 136 placebo) against disease of any severity and across all 15 identified strains, while protection against moderate to severe disease was calculated to be 77% (9 vaccine vs. 36 placebo).
In the same age group, CVnCoV provided 100% protection (vaccine 0 vs. 6 placebo) against hospitalization or death, said the vaccine developer.
Meanwhile, in participants above 60 years, who represented 9% of the analyzed cases, the available data did not enable a statistically significant determination of efficacy, admitted CureVac, with it adding that the data confirmed the favorable safety profile of CVnCoV in all age groups.
The study will continue to complete follow-up analyses for trial participants. Available data have been communicated to the EU regulator, said the firm.
“In this final analysis, CVnCoV demonstrates a strong public health value in fully protecting study participants in the age group of 18 to 60 against hospitalization or death and 77% against moderate and severe disease – an efficacy profile, which we believe will be an important contribution to help manage the COVID-19 pandemic and the dynamic variant spread,” said Dr Franz-Werner Haas, CEO, CureVac. “In the current context of an increasingly diverse environment of COVID-19 variants, and with very little residual prevalence of the original strain, we are confident that the HERALD study offers clinically relevant data regarding the effect of emerging variants on vaccine efficacy.”
The EU Commission, back in November 2020, announced a supply agreement with CureVac for 225 million initial doses of its vaccine with the option to extend that by a further 180 million doses, once the vaccine "has proven to be safe and effective against COVID-19."