Patient deaths trigger clinical hold in US, Bellicum awaits instruction

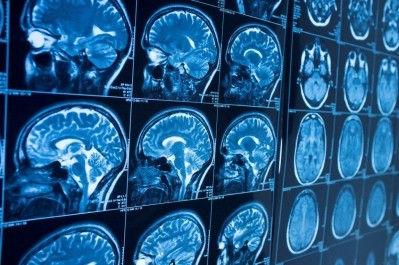
Bellicum’s lead candidate is an allogeneic T-cell therapy indicated for treatment in children and adults following a T-depleted, haploidentical hematopoietic stem cell transplant (HSCT), but three cases of encephalopathy has led the US Food and Drug Administration (FDA) to halt its clinical research.
The Texas-headquartered firm said encephalopathy – a general term to describe brain dysfunction – in three of its patients has been deemed “possibly related” to BPX-501 by the FDA.
Potential confounding factors in these patients may include prior failed transplants, immunodeficiency, and infection, said Bellicum.
The company said it is awaiting instruction from the regulator before determining the future of its lead candidate, and “will work closely with the FDA to address their questions.”
The hold does not affect the ongoing BP-004 registration trial in Europe for children with haematological cancers or orphan inherited blood disorders, in which BPX0501 is administered after initial allogeneic HSCT, the firm said.
December results
In December last year Billicum announced encouraging results from a BPX-501 trial in children with blood cancers and non-malignant diseases.
“These data show that administering BPX-501 cells following a haplo-HSCT may result in improved immune recovery and infection control, addressing significant risks in children undergoing a stem cell transplant who do not have access to a matched donor,” said Billicum at the time.
“Adding BPX-501 to a haplo-HSCT has the potential to improve outcomes and to make the curative benefits of transplants available to more children with cancers and genetic blood diseases,” study author Pietro Merli added.
T-cell troubles
T-cell therapy candidates have attracted increased attention from regulators following deaths in clinical trials.
In July, 2016 the FDA ordered Juno Therapeutics halt its ROCKET trial for JCAR015 – indicated to treat patients suffering relapsed or refractory B cell acute lymphoblastic leukemia – following three patient deaths.
Unlike BPX-501, Juno’s candidate uses patients’ own T-cells that have been genetically modified to express a chimeric antigen receptor (CAR), and bind to leukemia cells.
In November that same year, Juno announced two more deaths in the ROCKET trial due to swelling of the brain.
In September last year, the FDA placed Cellectis SA’s trials for cell therapy UCART123 on clinical hold following the death of a patient, who suffered from Cytokine Release Syndrome (CRS), a lung infection, and Capillary Leak Syndrome.