Product recall after mechanism failure complaints
Teleflex recalls faulty nasal delivery devices used to administer opioid overdose meds

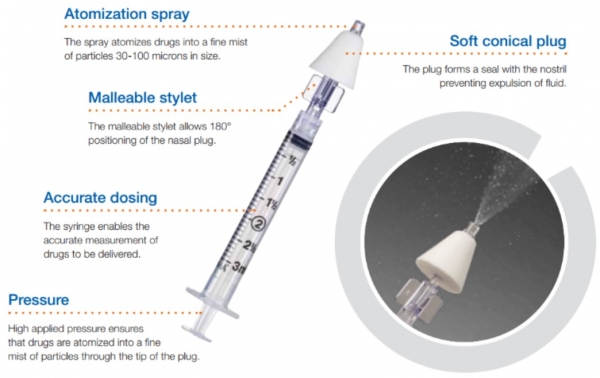
Teleflex withdrew 32 lots of its LMA intranasal Mucosal Atomisation Devices (MAD) in the UK and US in response to reports they to not release formulations as a spray, but instead a ‘straight stream’, making absorption less effective.
The firm said the faulty nasal spray devices could ‘result in serious injury or death’ in emergency situations – including the needle-free delivery of drugs to reverse life-threatening hypoglycaemia or epileptic seizures.
Both the Medicines and Healthcare products Regulatory Agency (MHRA) and the Food and Drug Administration (FDA) have been notified of the recall.
Defective drug dispersal
The devices in question are dependent on a mechanism which disperses formulations to patients as an atomised intranasal spray, but have instead been reported to release a straight stream – which is problematic in insuring uptake of APIs via mucous membranes.
The tech is designed to eliminate the risk of needlestick injury, as it avoids having to find and correctly access a vein for injection.
For this reason, the devices are also used by emergency services in the US to deliver naloxone hydrochloride to people who have overdosed on opioids.
This use can be for generics or brands (e.g. Narcan), vials of which can be assembled with Teleflex LMA MAD Nasal device as non-FDA approved improvised naloxone kits.
US Shortage
Since the US recall, Dublin-based competitor Adapt Pharma Ltd. – manufacturer of the FDA-approved Narcan Nasal Spray – has said it was aware of reports of a potential shortage of such kits. Thom Duddy, of Adapt Pharma, told in-Pharmatechnologist.com:
"Several departments of health from separate US states, community-based organizations, and other groups involved in naloxone distribution have turned to NARCAN Nasal Spray due to the recall of Teleflex’s MAD 300 Atomizer."
This is problematic for some emergency responders, who would otherwise require training for sterile techniques. Duddy went on to claim that Adapt Pharma is therefore partnering with different US organisations to "help combat this public health crisis."
Other customers of Teleflex Medical include Dutch biotech Mucosis B.V., which is using the Teleflex VaxINator MAD device to deliver a phase I trial vaccine for respiratory syncytial virus (RSV). However, this particular Teleflex intranasal device is not affected by the recall.
Teleflex Medical declined to comment.









