Anti-PCSK9 approvals a boon to Protein A supplier Repligen, says analyst
PCSK9 inhibitors are monoclonal antibody which work by blocking the enzyme PCSK9 from reducing the number of receptors on the liver that remove low-density lipoprotein (LDL) cholesterol from the blood.
In July, the US Food and Drug Administration (FDA) approved Sanofi and Regeneron’s drug Praluent (alirocumab), the first treatment in this new class of cholesterol-lowering drugs and a month later, a second PCSK9 inhibitor – Amgen’s Repatha (evolocumab) – received the regulatory thumbs up.
While the drugmakers and a proportion of the 74 million US adults who suffer from high cholesterol are likely to benefit from these approvals, so too will Repligen Corporation, according to Jefferies analyst Brandon Couillard.
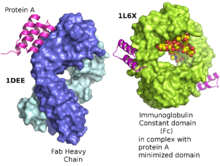
Repligen is a developer and producer of high-value consumable products used in the bioprocessing industry, including Protein A which is a critical reagent in the purification of monoclonal antibodies.
“Given Repligen’s envious position as the only global supplier of critical Protein A affinity ligands needed to purify mAbs, rising new approvals add visibility to its growth trajectory. The recent approval of two PCSK9s could be material over time.”
Furthermore, Couillard noted the FDA approved “a record eight new monoclonal antibody drugs in 2014 and another 350+ are in development,” further fuelling demand for Repligen’s consumables.
Expanded Opportunities
Couillard’s comments reflect those of CEO Tony Hunt who told investors during a conference call last month the surge in mAb approvals was a windfall for his firm:
“Over the past few months there has been a great deal of momentum in this market. This includes highly anticipated regulatory approvals and monoclonals targeting PSK9 to lower cholesterol and good progress for biosimilar version of the block set monoclonals Remicade, Rituxan and Humira,” he said, discussing his firm’s Q2 results (transcript here).
“In short the expansion of the biologics market also expands the opportunities for our products to deliver important product efficiencies and cost savings to biopharmaceutical manufacturers worldwide.”
Product revenues for the firm’s second quarter, ending June 30, were $21.5m, up 39% on the same period 2014, attributed to increased demand for Protein A affinity ligands and the firm’s ATF (Alternating Tangential Flow) system – a fermentation cell retention technology acquired in 2014.