Could umbilical cords hold the key to treating diabetes?
At Advanced Therapies 2023, we caught up with chief scientific officer Lindsay Davies to discuss the unique treatment – which is able to delay the disease from progressing.
BPR: Could you give me an overview of your role at NextCell?
I'm the chief scientific officer at NextCell in Stockholm, Sweden and it's my responsibility to develop the strategy for research and development, process development, and developing our portfolio of products including working with our clinical trials.
BPR: Could you tell me a little bit about your drug candidate ProTrans? I understand it’s currently in phase 2 development.
Yes, we’ve just finished phase 2 development for type 1 diabetes. The treatment is a mesenchymal stromal cell product that’s derived from umbilical cord tissue. We can take the tissue and culture it in the laboratory and by isolating the cells from the tissue, we can expand them and generate therapeutic doses for a cell therapy.
ProTrans has a few unique selling points to it, compared to other mesenchymal stromal cell products. For example, the company has a patented selection algorithm, so we can choose the best donors based on their ability to modulate the immune system, depending on the therapeutic indication that we’re going to use the cells for. We’re also able to pool donors, so we choose five different donors selected using the algorithm and combine together to generate a batch of product. This allows us to reduce the batch-to-batch variability that you can see with other products, but also give us scalable proportions for a commercialised product.
The cells themselves are then cryopreserved, so we can keep them in the freezer. It’s an allogeneic therapy, so we don’t have to match the cells to the patients the way you would for tissue matching.
So the product can be thawed at bedside, it’s ready, it's in the freezer and it has an extended shelf life, so it’s there for years. We also have a fixed dose –unlike other MSC products, where they dose on the patient's body weight, which means that, even if it is cryopreserved, they have to thaw it and formulate it for that particular patient, which requires a facility to do that for them, before they can give it to the patient. With our fixed dose, there is no need for that, you can literally thaw it at the bedside.
BPR: So you don’t need to partner the therapy to a specific patient? How does the criteria work?
No. The cells themselves modulate the patient's immune response. So in the case of type 1 diabetes, they are modulating the autoimmune response that's causing their pancreatic beta cells to be destroyed. At the moment, we are treating patients who are newly diagnosed with type 1 diabetes, which means that they still have their own insulin production to some degree, but their cells are being destroyed. That means year on year they need more and more insulin in order to maintain a normal glycemic control of their sugars. With this treatment, we can slow the progression of the disease by stopping the pancreatic beta cell destruction.
Patients come in for a single 40 minute treatment; they are infused through a drip and can then go home without any serious adverse side effects. Our data suggests that this single treatment can halt the disease for at least 1 year, and thereafter the progression of the disease is significantly slowed in comparison to individuals receiving insulin replacement only. As the disease progresses individuals with type 1 diabetes are more at risk of complications like heart disease and poor wound healing so by slowing the disease we are also reducing the risk of such illnesses
BPR: So the treatment is designed to prevent the diabetes from deteriorating further?
Yes. ProTrans won’t reverse it or regenerate the pancreas, because the cells don't engraft into the patient, they don't stay around. They're infused in, and they have what we call a domino effect, so they interact with the patient's immune system to have a long-term therapeutic effect, but the cells themselves are lost from the patient, they're cleared away. So there is no significant risk of any tumors.
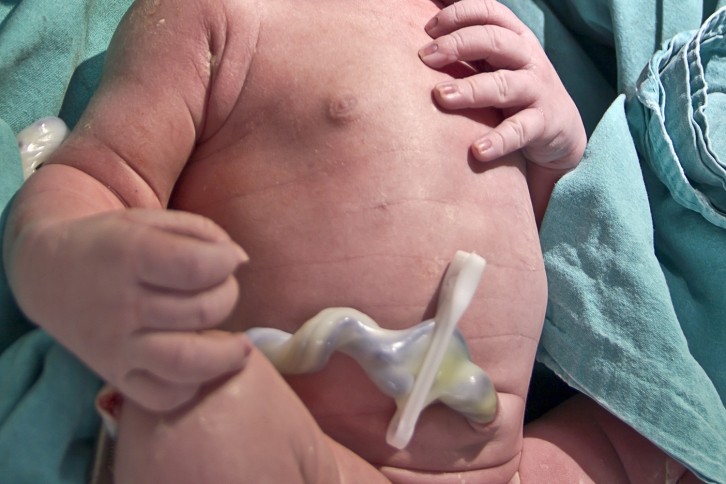
BPR: How are the umbilical cords extracted? Do you take them straight after child birth?
Umbilical cords contain tissue that is normally thrown away – so there is a lot we can use! It's not like having a bone marrow aspiration, so there is no pain associated with donation. We take them straight after childbirth and transport the cord to the lab for processing. The cells isolated from the umbilical cord can be used allogeneically and because the cord comes from the baby, not from the mother when it develops, it means these cells are very young. Also, because of the fetal maternal tolerance that you have when a baby's growing, the cells are already educated to deal with the immune system because the mother and the baby don't have the same genetic makeup. So they're an ideal source of mesenchymal cells for allogeneic therapies.
BPR: How is it logistically to collect those cells from the umbilical cords?
It’s very easy because you can literally take the whole cord and cut it up. We put the whole cord as tissue slices onto a culture plate and the cells themselves will move out of the tissue and start growing. So you don't need to treat them with different enzymes or anything like that. They move out of the tissue once they're cultured onto plastic and then we can take those cells and we can expand them.
BPR: When the baby has been born, does the cord need to be preserved? What is that process?
So we go straight to the clinic and we take the cord, but the cord itself can last a couple of days before use. It just goes straight into a solution so that it stays in a hydrated, and then it can be transported to the lab.
BPR: As you are currently in phase 2, when do you anticipate moving into phase 3 and what is the timeline for this treatment?
Our phase 2 data is currently in adult patients, so we’ve worked with patients that are 18 to 40. However, it's important for us that when we go for commercialization of the technology that we're able to treat children as well, because a lot of type 1 diabetes is diagnosed in early teenage years. So, we just finished a phase 1 and we’ve just started a phase 2 trial in pediatric patients. We’ve had discussions with the European Medical Agency about the pediatric investigational plan that's needed with a drug product that's used for children. On completion of our phase 2 in children next year, we will move towards phase 3 trials and we’ve already commenced discussions with EMA about that.
Now, we're at that stage where we're looking for partners to scale up our production for commercialization applications, and also for our phase three clinical trials.
BPR: So your adult and pediatric trials are working alongside each other?
We would like to conduct phase 3 trials that cover the whole patient population of newly diagnosed paediatric type 1 diabetics through to adults. So, because of the safety implications of working with children, we're waiting for that phase 2 data to make sure that we have efficacy and safety in that patient cohort before we move to phase 3.
This is a platform technology for us. So although we've really focused on type 1 diabetes as our primary indication, we also have phase 2 trials in virally induced respiratory disorders. We've been treating patients with COVID who have severe pneumonia and slowing the disease progression so they’re not taken into intensive care or put onto a ventilator. We’ve also been working with other viral respiratory disorders like influenza A, RSV and HMPV. Our platform technology really allows us to be able to go into other clinical indications as well – which is very exciting.