Production begins at uniQure MA plant to support gene therapy pipeline
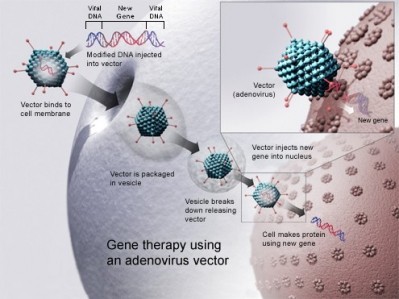
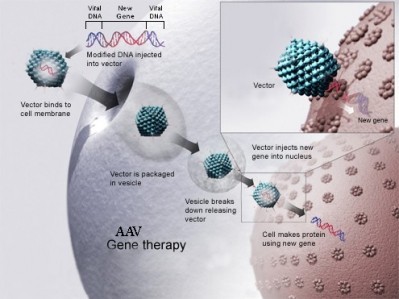
The Netherlands-based biopharma firm’s lead product is Glybera (alipogene tiparvovec), a treatment designed to compensate for lipoprotein lipase deficiency (LPLD), which became the first gene therapy to achieve regulatory approval in Europe in 2012.
Uniqure uses insect cells and baculoviruses to manufacture Glybera and other AAV-based gene therapies in its pipeline from a site in Amsterdam, but following an investment in in 2013, the firm has begun producing research batches at a second manufacturing site in Lexington, Massachusetts.
“The site is not approved yet by regulatory authorities but uniQure expects the first EU GMP approval by mid-year 2016,” a spokesperson from the firm told Biopharma-Reporter.com.
“We are producing research batches on 50L scale. The first 500L cell growth runs have been successfully executed.”
The site is fully single-use, and while uniQure was unable to be too specific on the disposable processing technologies being used, we were told the firm is using stirred tank reactors being run at 500L scale with the option to expand to 2,000L scale if needed.
Supporting Bristol-Myers Squibb
October hailed a milestone for uniQure and gene therapies in Europe, with the Charité University Clinic in Berlin, Germany announcing the treatment of the first patient with Glybera since the therapy’s approval.
However, speaking last week to discuss third quarter results, CEO Joern Aldag said the firm is not pursuing US regulatory approval for LPL deficiency “mostly due to the additional clinical studies that the FDA indicated will be required.”
The plant will not be, therefore, be used exclusively for Glybera but rather “has been designed as a multi-product facility to meet the needs of the entire pipeline of products in development,” including efforts to support the potential $2bn (€1.8bn) partnership with Bristol-Myers Squibb signed in April this year.
At the time, uniQure’s CEO Joern Aldag said it was “one of the largest and most comprehensive collaborations in the gene therapy sector to date.”
To date, BMS has paid uniQure a total of $140m through the agreement, including $68m in the third quarter ending September 30, and has acquired 9.9% of the company.