GE looks to single-use modular BioParks to relieve capacity investment risk
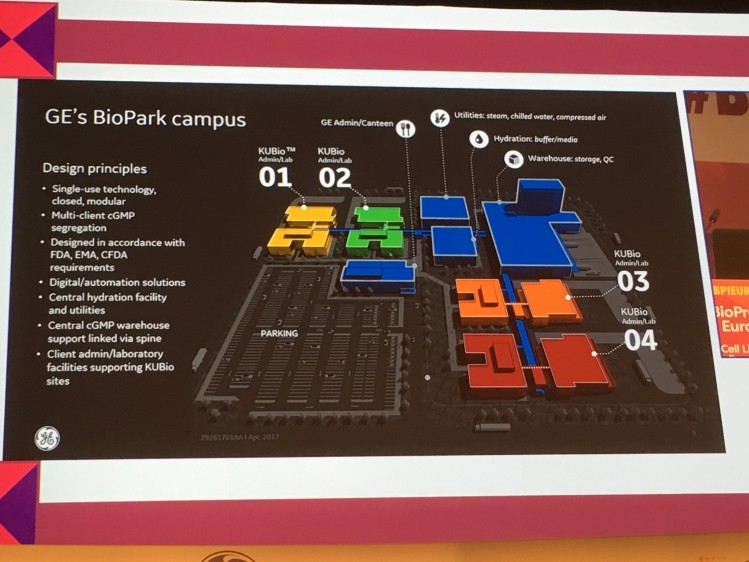
Last September, GE Healthcare announced it was constructing four off-the-shelf KUBio facilities, equipped with single-use bioprocessing equipment forming a €150m biomanufacturing campus in Ringaskiddy, Ireland.
GE’s David Radspinner said at last month’s Bioprocessing International (BPI) European Summit in Amsterdam a shared vendor run modular campus build on the benefits of single-use facilities and help drugmakers further minimise their investment risk.
“There’s a whole range of challenges [biopharma] faces in regards to capacity,” Radspinner – who heads GE’s new BioParks division – told delegates, but the decision to buy or build a plant, use a CMO or modify an existing facility is a decision that costs hundreds of millions of dollars.
Such a significant investment needs to be made “as late as possible” in the development process while ensuring production is ready for a product’s approval, he continued.
“There can be a tremendous improvement in reducing time to get to market with the right technologies and processes, and that’s worth many, many, millions of dollars,” he said.
“In a classical stainless facility that decision typically is made 4-5 years before launch. Moving towards single-use, you can push that off to around two-and- a-half years. In terms of the economics, how much is that year and a half worth?”
And GE itself has pushed this further still, creating facilities from start to finish in 18 months through its FlexFactory single-use and KUBio modular platforms.
He argued not only could this be more valuable than cost per gram benefits from stainless steel systems, but also reduces the risk of losing out if a molecule fails in late-stage development or on the market itself.
While GE is yet to announce a tenant, Radspinner said the Irish BioPark is expected to be the first of several rolled out globally as customers look to combine the benefits of a shared campus – cheaper utilities, warehousing services and buffers/liquids – with the flexibility of modular plants.