OTCQB: PMCB
Cell-in-a-Box: PharmaCyte seeks FDA advice for inoperable cancer trial

California based PharmaCyte Biotech Inc is looking to initiate human trials for the cell encapsulation technology to treat locally advanced inoperable pancreatic cancer (LAPC).
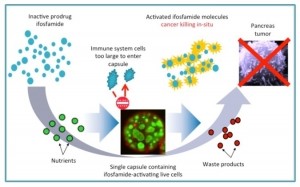
Manufactured at a facility in Thailand, Cell-in-a-box for LAPC is genetically modified liver cells contained in semi-permeable capsules, used to activate the cancer prodrug ifosfamide.
Acting as an “artificial liver”, the technology allows a lower concentration of the chemotherapy drug to be used. This minimalises off-target cytotoxicity for a more precise release of the drug at the tumor site.
Kenneth Waggoner, CEO of PharmaCyte, told Biopharma-Reporter “When the ifosfamide comes in contact with the encapsulated cells, those cells act as an artificial liver and activate the chemotherapy drug at the source of the cancer.”
The firm has planned a meeting on January 17 with the US FDA’s Center for Biologics Evaluation and Research (CBER) to evaluate how the IND application may proceed.
Waggoner added “Because our cell therapy is a biologic, the requirements are quite stringent. We’ll know a great deal more after we have our pre-IND meeting with the FDA.”
Living Capsules
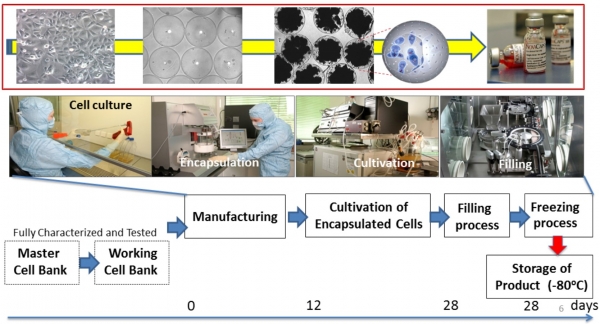
PharmaCyte manufactures these cell-in-a-box therapies under cGMP standards with its supplier, Austrianova, at a facility in Bangkok, Thailand.
The Cell-in-a-box tech uses a suspension of living cells in a proprietary polymer solution – Polymer A – with sodium cellulose sulfate.
In a multi-step process, the suspension is passed through a special instrument to produce micro-droplets. These are dispensed into a bath of a second solution – Gel8 – which interacts with the first polymer to create a semi-permeable membrane surrounding each cell. Each capsule is around 0.7 – 0.8mm diameter and can hold up to 10,000 liver cells.
Waggoner explained that cellulose is preferable to seaweed derived alginate for capsule durability: “When the alginate capsules degrade, the immune system attacks and kills the cells inside. Our capsules do not cause damage to surrounding tissues while in the body for more than two years.”
The Cell-in-a-box therapies can be frozen to -80˚C and shipped frozen to the study sites: “[PharmaCyte’s] encapsulated cells can be stored frozen for more than five years and recovered with more than 95% viability upon thawing. Alginate capsules cannot.”
Waggoner told us the firm already has a cold chain logistic partner which has been shipping frozen encapsulated cells to the US, Singapore, and to sites in Europe for the last couple of years.
Pancreatic cancer trial
The proposed trial of Cell-in-a-box for LAPC will compare efficacy of the ifosfamine-based liver cell therapy to other chemotherapies Abraxane (paclitaxel) and gemcitabine.
Waggoner explained that the Cell-in-a-box capsules are implanted as close to the patient’s cancerous tumor as possible. The prodrug that is normally activated in the liver (ifosfamide) is then given intravenously at one-third the normal dose.
“This ‘targeted chemotherapy’ has proven effective and safe to use in past clinical trials and this novel form of chemotherapy results in no side effects using low does ifosfamide.”
Specifics on how long the cellulose capsules remain intact in the human body are not yet known, with Waggoner adding “That’s because none of the patients enrolled in two human clinical trials remained alive long enough.”
However, the firm does “know the capsules remained intact, with cells still alive and functioning after having been in patients for more than two years.”
For these reasons, the firm outlined plans to seek advice on applying for the clinical trial for LAPC in a statement: “PharmaCyte has submitted a list of important questions to the FDA that will be essential to the design of our trial and how it is to be conducted.”
Austrianova
Waggoner told us that although Pharmacyte and its Singapore based partner Austrianova are not formal partners, PharmaCyte does own 14.5% of the equity interest of the parent company of Austrianova SG - Austria Private Limited.
There is also significant cross over in board members, however, Waggoner added “The success of Austrianova is interdependent with the success of PharmaCyte in numerous ways.”
“The particulate cellulose sulfate Austrianova uses is a proprietary cellulose sulfate that has been patented and licensed to Austrianova,” Waggoner explained.
“We are the main customer, and Austrianova is the only supplier for the microcapsules we use in our cell therapy.”