BIO 2015
Vaccines for mothers: FDA talks stumbling blocks on new pathway
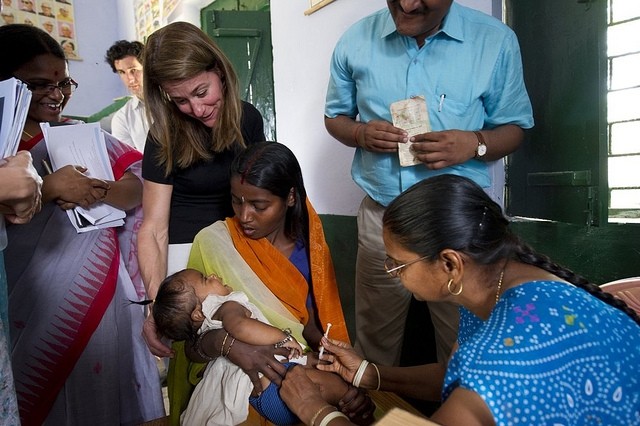
Vaccination of pregnant women grants infants immunity during the few months after birth when babies are susceptible to infectious disease but too young to receive most vaccines: 44% of deaths from pneumonia in under-fives, for example, occur in the first month of life. Apart from the BCG injection, which is given at birth, routine vaccination of infants begins at two months.
The Bill and Melinda Gates Foundation is encouraging the development of vaccines for influenza, RSV (respiratory syncytial virus), pertussis, GBS (Group B Streptococcus), and tetanus, to be given to mothers to protect their children. But the regulatory pathway for vaccines for pregnant women is unclear, according to the Foundation’s Senior Programme Officer Ajoke Sobanjo-ter Meulen during a panel at BIO 2015 this week.
Maternal vaccines have extra regulatory hurdles to jump, because of rules about clinical trials on pregnant women and because the products are intended to benefit children, not their mothers.
Trial rules
Under FDA rules, if the vaccines are aimed at infants, sponsor companies do not need to show a clinical benefit to mothers. However, safety is a major concern for products given to pregnant women, and extra rounds of testing are needed in clinical trials, said FDA Director of Vaccines Marion Gruber, also speaking at BIO 2015.
As a matter of course, companies wishing to license a drug or vaccine for mothers-to-be must complete Phase I and II studies in non-pregnant women, before beginning clinical studies again in pregnant patients. But the first round of Phase I-II studies can be skipped if the drug is licensed for the general population.
Animal reproductive toxicity studies are also required before vaccines can be approved for expectant mothers.
Pregnancy: what’s a ‘complication’?
When sponsors do come to perform clinical trials on pregnant women, regulatory issues remain. Even “low-risk” pregnancies can incur complications, and studies need to control for adverse events not linked to the vaccine.
The number of participants necessary in clinical trials is also up for debate – the most common question from companies, according to Gruber.
“The factors that need to be considered here include what are the safety data that are accrued with the vaccine? If it is a vaccine that is already licensed, such as a [flu] vaccine, the safety database likely doesn’t have to be as large.”
Beyond regulation
Once the regulatory pathway is clear, other stumbling blocks remain for would-be makers of maternal vaccines.
Strain selection and manufacturing of vaccines tend not to be tailored for tropical and developing countries where the immunisations are most needed, said Sobanjo-ter Meulen.
Recouping investments are also particularly tricky in this area, added Lou Fries, Chief Medical Officer of vaccine-maker Novavax. He said his company is picking maternal vaccines to develop by looking for endpoints that will reap revenue in higher-income countries while also meeting a meaningful need in the developing world – “a challenge” the WHO and FDA’s CBER are advising on.
Once these vaccines are licensed, demand from more developed countries will soon be outstripped by lower and middle income economies, so companies need to project production needs and balance their capacity, he added.
Vaccines for mums: progress so far
Tetanus immunisation programmes for expectant mothers have been occurring for several years in the developing world and have given a safety and efficacy record to maternal vaccinations. Pregnant women are also routinely immunised for influenza in many high and low-income countries.
However, the pertussis vaccine, although widely used, proved “a disaster,” due to severe local reactions to a whole-cell (wP) formulation, says the Bill and Melinda Gates Foundation’s Keith Klugman. Programmes switched to an acellular vaccine which has proved less effective.
The Foundation’s most advanced programme in this area is for RSV. “We have a proof-of-concept, we have an F-protein monoclonal [antibody] which has been very successful. So the question is, can we move on to using F-protein in pregnant women?”
GSK has revealed it is developing maternal vaccine candidates for RSV and GBS.