Novartis to bring biomanufacturing ‘all under one roof’ with $1bn Sandoz investments

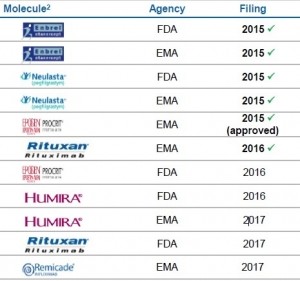
Through its subsidiary Sandoz, the Swiss Pharma giant has placed itself among the major players in the biosimilar market. The firm has a number of copycat biologics available in Europe and last September was the first to launch a biosimilar in the US – a version of Amgen’s Neupogen (filgrastim), Zarxio.
And during a media day at the firm’s facility in Schaftenau, Austria, Sandoz announced it intends to launch five more oncology and immunology biosimilars in Europe and the US over the next four years.
However, the firm’s global head of biopharmaceuticals Carol Lynch said, “having a product and being able to commercialise it isn’t enough, you really need to have access to capabilities across the value chain.
“Investment is required from a manufacturing perspective to make sure that we can really have that capacity and high quality and reliability in the supply chain that is needed to be successful.”
$1bn injection
The Schaftenau site recently completed a €150m expansion which added a biopharmaceutical fill/finish and packaging facility capable of filling around 300 prefilled syringes per minute, but this represents just 15% of what Novartis says it is spending to boost capacity for both innovative biologics and biosimilars in Austria.
Lynch said this heavy internal biomanufacturing investment is intended to bring “all of our biologics manufacturing under one roof, figuratively” to ensure the firm has the capacity required to meet the needs of the market into the future and ensure flexibility between its original biologics and biosimilar portfolios.
“Thus here in Austria between this site and the one down the road in Kundel, between 2010 and the end of the decade $1bn will have been invested to support the expansion of manufacturing capacity which we know is required for a biologics portfolio,” she told the delegate of journalists.
“This area is not for the faint of heart. You really have to have the commitment and the willingness to financially commit for the long-term.”
Contract manufacturing
Sandoz did not reveal details of its manufacturing capacity in Austria or worldwide when asked by this publication, due to “competitive reasons” but Lynch did tell us that while Novartis currently uses contract manufacturing organisations (CMO) across its biologics network, the firm’s objective is to be self-sufficient in the future.
“It’s fair to say that given the uncertainty about how these markets are going to evolve we shouldn’t rule out that we may choose to use contract manufacturers in the future, but in terms of building our plans, our goal is to contain capacity in-house.”
A number of Big Biopharma companies have said they are following a similar strategy of building in-house biomanufacturing capacity. Novo Nordisk, for example, has been vocal about investing internally and looking to minimise its use of CMOs, as has AstraZeneca.