Looking for a single-use modular biologics plant in Ireland? Talk to GE
The bioprocessing technology and services subsidiary of General Electric announced yesterday it was constructing the four off-the-shelf facilities as part of a biopharmaceutical manufacturing campus on Industrial Development Agency (IDA) Ireland’s site in Ringaskiddy, County Cork.
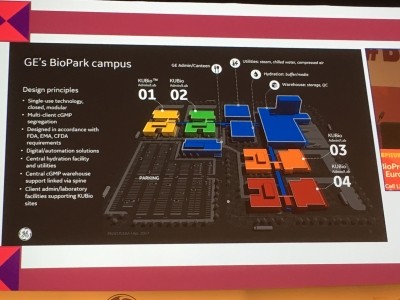
“GE BioPark Cork will be a GE-managed campus including four fully-equipped off-the-shelf KUBio factories owned by independent biopharma companies manufacturing proprietary medicines, with GE running centralised shared utilities and site services,” the BioPark’s general manager David Radspinner told Biopharma-Reporter.com.
Around 100 people will be employed by GE on the site to support the four plants, though a further 400 will be employed by the biopharma firms which buy the facilities.
“We are in the process of in-depth discussions with a number of companies who are very interested in the concept of BioPark and KUBio in Europe,” Radspinner said.
“The rapid construction time for KUBio is a very attractive proposition, as it will help companies get their products to market more quickly than a traditional stick built facility.”
KUBio
GE’s modular platform KUBio, he continued, enables construction and assembly of a cGMP facility fully equipped with single-use technologies within 18 months – about half the time of a traditional biomanufacturing plant.
The firm recently completed the world’s largest modular antibody production plant for JHL Biotech in China using the KuBIO platform, and in June inked a $350m deal with Pfizer for a KUBio biomanufacturing centre in Hangzhou.
But the planned investment will be the first examples of GE’s modular biomanufacturing platform in Ireland, which has become a major bioprocessing region through major investments from companies including Shire, Pfizer, Bristol-Myers Squibb, Eli Lilly and Merck & Co.
Supporting Ireland
In a double boost for Ireland’s bioprocessing business, GE also announced it creating a single-use Centre of Excellence at the National Institute for Bioprocessing Research and Training (NIBRT) in Dublin.
NIBRT – described as a “flight simulator for biopharma manufacturing” – is a government-funded centre founded in 2011 and, according to Radspinner, has since trained almost 4,000 bioprocessing professionals.
“Some manufacturing sites in Ireland are already working with single-use systems and we believe this technology is poised for enormous growth in Ireland. GE's investment will help catalyse the rapid development of the sector in Ireland and globally.”
In a statement, NIBRT’s CEO Dominic Carolansaid the centre is: “delighted to partner with GE on the next generation of bioprocessing equipment, which will accelerate the introduction of these new technologies to the biopharmaceutical manufacturing industry, helping to reduce manufacturing costs and increase the access to these valuable therapies.”