'First Japanese Clinical Trial using Stem Cells’ isn’t and doesn't say Researchers
When news of the so called 'clinical trial' broke earlier this month most reports stated the project was first-of-its-kind effort focused on the development of stem cell-based treatments for patients suffering age-related macular degeneration (AMD).
The trouble is that this is not what is actually being done according to Dr Masayo Takahashi of the Riken Center for Developmental Biology and the Institute of Biomedical Research and Innovation Hospital in Kobe, Japan where the research is being conducted.
Dr Takahashi told BioPharma-Reporter.com the “immediate plan is not for a formal clinical trial that could be used to apply for marketing approval for a medical product.”
Nor does the research involve harvested stem cells as has widely been reported.
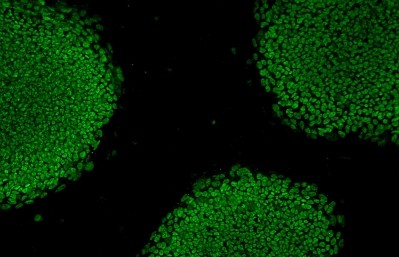
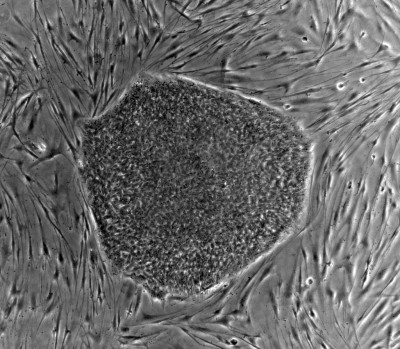
“The protocol does not call for harvesting of stem cells from the patients. Instead, the patient's somatic cells will be reprogrammed into iPS cells [induce pluripotent stem cells], which will then be differentiated into retinal pigment epithelium (RPE) cells grown in sheets. RPE cells are affected in wet-type AMD, and the idea is to use these RPE cell sheets for autologous transplantation.”
Growth
The team will grow the iPS cells using standard culture techniques in a dedicated facility according to Dr Takahashi, who added that: “As the planned study is not a clinical trial that would lead to a medical product approval, it is not conducted under manufacturing conditions.”
Despite this, the team has taken great care to ensure it can select the most appropriate somatic cells continued Dr Takahashi, explaining that: “We perform a battery of quality assurance tests in collaboration with the Kyoto University Center for iPSC Research and Application.”
Kyoto University made the headlines last year Dr Shinya Yamanaka, head of the research center, was awarded the Nobel Prize for his work on iPS cells.
Ultimately, Dr Takahashi’s team plan to transplant the cells into AMD patients as tissue grafts and are already working on a process to ensure that no iPSCs are included in the iPSC-derived RPE grafts intended for transplant.