Dispatches from CPhI
'The sexiness of the ADC world' - CMOs talk antibody-drug hybrids at CPhI

Biopharma-Reporter.com was at CPhI in Frankfurt, Germany and - like an industry-targeting antibody attached to a highly potent microphone - we sought out the experts on one of the hot topics dominating this year's event, antibody-drug conjugates.
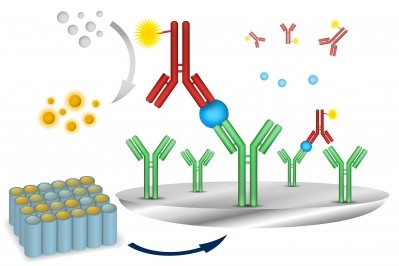
The bringing together of small molecule cytotoxics with antibodies and conjugating them together with the correct linker technologies in order to produce the next generation of cancer drugs, was an exciting concept, amongst the players we spoke with, with ADCs being described as everything from “groundbreaking” to “sexy.”
It is no surprise, therefore, that Big Pharma has been keen to get involved, and in the last few weeks Roche, Novartis, Eli Lilly and AstraZeneca - through the acquisition of Spirogen - have all invested in ADC technology.
Where Big Pharma goes, contract manufacturers have followed and there has been a flurry of ADC investment amongst CMOs of late with Carbogen Amcis and SAFC both entering the market. We asked Dr. Andreas Weiler, Head of Strategic Marketing at SAFC, with Big Pharma investing in its own technology, where are its ADC customers likely to come from.
“80% [of the innovation] right now takes place on the smaller players, the virtual pharma companies and small biotech, and 20% takes place in the Big Pharma companies,” he said.
“ADCs were so sexy for the Big Pharma guys as they had an arsenal of antibodies and they could use this arsenal to now link it to a proven technology and that’s why a lot of Big Pharma guys are very active in late phase clinical projects.”
However, he continued, “there’s a new era now with new innovative biotech companies developing new linkers, new toxins, and that’s where you need to be.
“Right now the majority of the late phase ADC drugs are owned by Big Pharma and that may continue, but there may be a shift very soon with more innovative companies coming in. Spirogen was acquired just a couple of days ago by MedImmune (AstraZeneca) and that may be the path for many of these innovators.”
CoMpetitiOn
Piramal has been offering ADC services for a number of years and recently upgraded its manufacturing capacity at its Grangemouth, UK, facility. President of API Services Mark Cassidy told us with the new players entering the sphere, “if you’re scared or worried of having more than one competitor you’re in the wrong industry segment.”
He continued: “We’re talking about a relatively limited number of competitors out there but hopefully there’s exciting pipelines. Competition is good in the sense that it stimulates more companies at looking at solving the problems.”
One of these competitors, Catalent Pharma Solution only entered the niche market in April when it licensed the SMARTag technology from Redwood Bioscience. Will Downie, Senior VP Global Business Development told us: “We have a technology here that will stand alone from the sea of ADCs that are out there at the moment. Time will tell, of course, if that’s the case or not.”