Gene therapy tech could be 'game-changer' for CNS biologics, says Medgenics
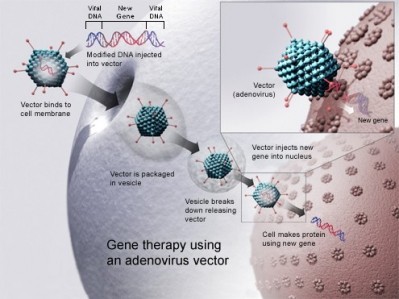
The company’s TARGT technology – previously known as BioPump – uses ex vivo gene therapy and a patient’s own tissue in order to produce and deliver therapeutic proteins and peptides.
Announcing first quarter 2015 results, Medgenics said it was expanding an ongoing collaboration with Harvard University to explore the viability and durability of its TARGT platform for potential applications involving the central nervous system (CNS).
While CEO Mike Cola admitted “these are pre-clinical experiments, they’re very early,” he acknowledged there is a need for successful interventions in the central nervous system to treat acute diseases that monoclonal antibodies struggle to target.
“In most cases most drug monoclonal antibodies do not cross - or do not cross in high concentrations - from the periphery into the central nervous system. So it's not possible to successfully treat some of these diseases with conventional therapy,” he told stakeholders.
“If we’re able to succeed here it would be a game-changer for many of these patients and certainly would provide a very exciting extension of our technology.”
The company also told investors the quarter had seen it file its first investigational new drug (IND) application with the US FDA for its lead candidate, MDGN-201, a TARGT- Erythropoietin (EPO) for treating renal anaemia.
The platform works by using the proprietary device DermaVac to harvest micro-organs from a patient’s abdominal dermis, which are then transduced in cassette bioreactors using HDAd vector to create TARGT-EPO. The HDAd vector is washed away, and the TARGT is implanted back into the patient where it produces endogenous EPO.
The continuous, autologous protein production technology, the company says, offers a number of key advantages over other recombinant protein therapies, including an improved durability in vivo, a greater cell uptake and less immunogenicity.